BMS Medicals Junala Verified
Manufacturer in China
30 results returned
-

Grasping pliers (three holes)
BMS Medicals
-

Grasping pliers (hollow vertical teeth)
BMS Medicals
-

Trocar device (titanium alloy with belt protection)
1 Variations
BMS Medicals
-

Trocar device (stainless steel without protection)
1 Variations
BMS Medicals
-

Biological clamp (with Cohui Biological clamp)
2 Variations
BMS Medicals
-

Dental Tips
BMS Medicals
-

Self Sealing Flat Pouch
BMS Medicals
-

Non-Woven Fabrics Bedsheet
BMS Medicals
-

Sterile Delivery Pack
BMS Medicals
-

Uniform Isolation Gown
BMS Medicals
-

N95 Face Mask(With/Without Valve)
BMS Medicals
-

Basic Dressing Set
3 Variations
BMS Medicals
-
Alcohol Prep Pad
BMS Medicals
-

Wound Adhesive Plaster
BMS Medicals
-

Cotton Self-Adhesive Bandage
BMS Medicals
-

Spandex Elastic Bandage
BMS Medicals
-

Gauze Ball
BMS Medicals
-

Wow Gauze Bandage
BMS Medicals
-

Vinyl Gloves
BMS Medicals
-
Disposable Syringe
BMS Medicals
-
Wound Drainage System
2 Variations
BMS Medicals
-
Luxury Urinary Drainage Bag
7 Variations
BMS Medicals
-
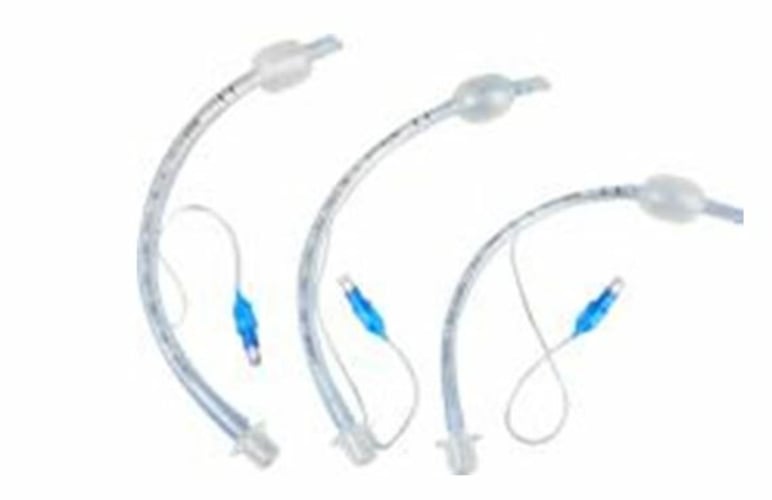
Oral/Nasal Endotracheal Tubes
2 Variations
BMS Medicals
-
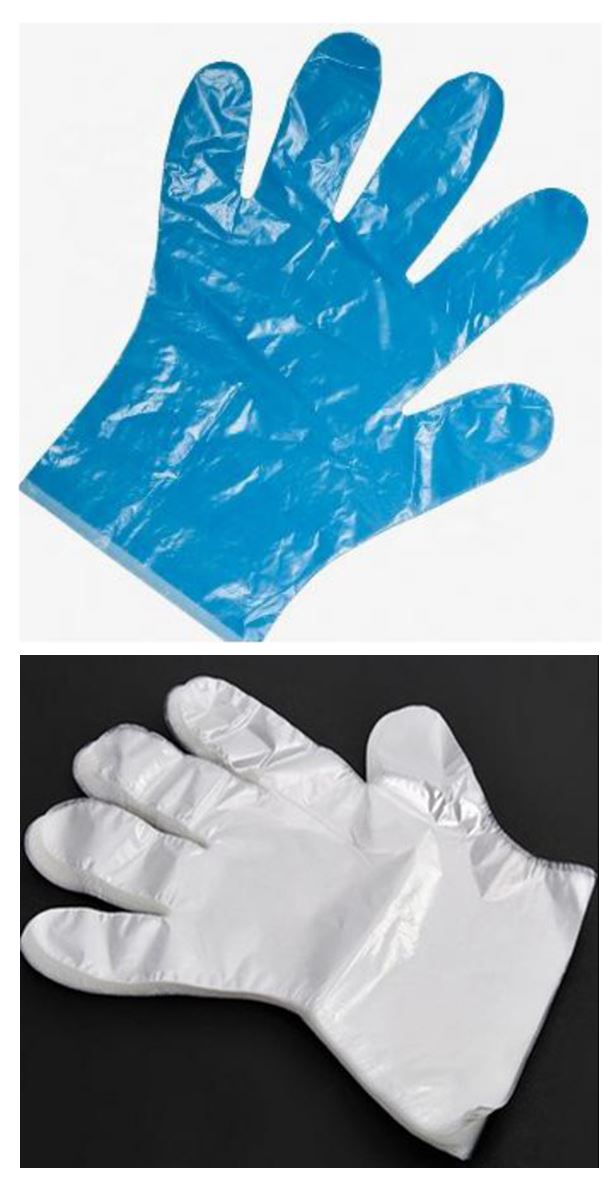
Disposable PE Gloves
BMS Medicals
-

Cotton Swab/Cotton Buds
BMS Medicals
-

Adhesive Zinc Oxide Plaster
BMS Medicals
-

Non Woven Cap
BMS Medicals
-

Gauze Bandage
BMS Medicals
-

Sterile And Non-Sterile Gauze Ball
BMS Medicals
-

Sterile Gauze Swab
BMS Medicals
Import License Not Issued
China 🇨🇳
Import License Not Issued
India 🇮🇳
Import License Not Issued
United States 🇺🇸
Import License
Indonesia 🇮🇩
Import License
Brazil 🇧🇷
Import License
Pakistan 🇵🇰
Import License
Bangladesh 🇧🇩
Import License
Nigeria 🇳🇬
Certification
ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
EN ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
93/42/EEC Annex II
COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices
Emergo: Global Medical Device Consulting