BMS Medicals Junala Verified
Manufacturer in China
30 results returned
-

Grasping forceps (alligator)
BMS Medicals
-

Grasping forceps (mesentery)
BMS Medicals
-

Grasping forceps (appendix)
BMS Medicals
-

Grasping forceps (Fine tooth two hole single acting)
BMS Medicals
-

Grasping forceps (duck bill)
BMS Medicals
-

Grasping pliers (blunt head)
BMS Medicals
-

Trocar device (titanium alloy without protection)
1 Variations
BMS Medicals
-

Trocar device (titanium alloy with belt protection)
BMS Medicals
-

Converter
2 Variations
BMS Medicals
-

Dental Cotton Rolls
BMS Medicals
-

Heat-Sealing Gusseted Pouch
BMS Medicals
-

Underpad
BMS Medicals
-

Surgical Hood
BMS Medicals
-

Nurse Paper Face Mask
BMS Medicals
-

First Aid Kit
2 Variations
BMS Medicals
-

Net Bandage
BMS Medicals
-

Cotton Swab
BMS Medicals
-

Prewashed Laparotomy Sponge
BMS Medicals
-

Disposable PAP Smear Kit
BMS Medicals
-

Two Fingers Cots
BMS Medicals
-

Latex Examination Gloves
BMS Medicals
-
Insulin Syringe
BMS Medicals
-
Disposable Blood Transfusion Set
2 Variations
BMS Medicals
-
Tracheostomy Tube
2 Variations
BMS Medicals
-

Silicone Resuscitator
BMS Medicals
-

Connecting Tube
BMS Medicals
-

Heat And Moisture Exchange Filters
2 Variations
BMS Medicals
-
Disposable Scalp Vein Set/Intravenous Needles
BMS Medicals
-
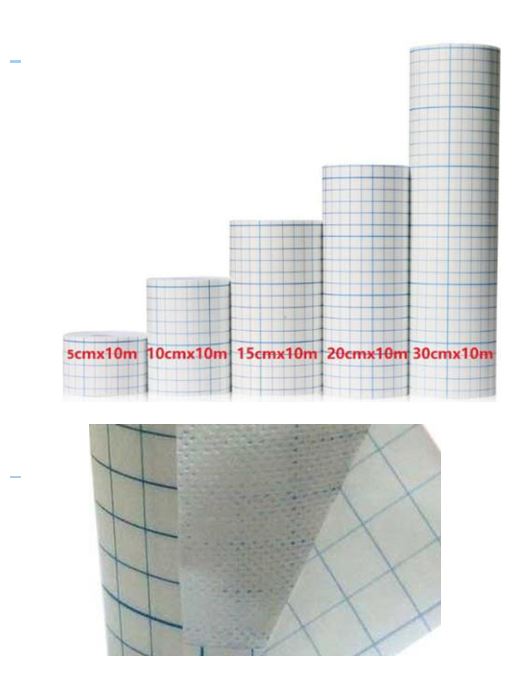
Non Woven Adhesive Dressing Roll/Tape
BMS Medicals
-

Triangle Bandage
BMS Medicals
Import License Not Issued
China 🇨🇳
Import License Not Issued
India 🇮🇳
Import License Not Issued
United States 🇺🇸
Import License
Indonesia 🇮🇩
Import License
Brazil 🇧🇷
Import License
Pakistan 🇵🇰
Import License
Bangladesh 🇧🇩
Import License
Nigeria 🇳🇬
Certification
ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
EN ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
93/42/EEC Annex II
COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices
Emergo: Global Medical Device Consulting